
New Treatment Paradigm
In Development
Epinephrine is the only drug approved for the emergency treatment of anaphylaxis. Epinephrine needs to be administered as soon as symptoms of a severe allergic reaction occurs. Failure to promptly administer epinephrine in the event of an anaphylaxis greatly increases the chance of hospitalization and has been associated with deaths.
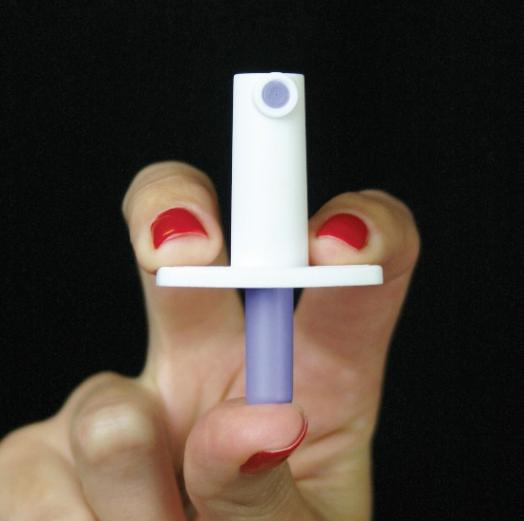
Insignis is developing an epinephrine sublingual spray formulation as a rescue medication for anaphylaxis. The liquid formulation, containing the FDA approved epinephrine prodrug Dipivefrin, is housed in a small, easy to carry and simple to use, UnitDose Spray device that at-risk patients can use at first signs of an allergic reaction when epinephrine is most effective in reversing symptoms of anaphylaxis.
Advantages of Liquid Sublingual Spray as a Rescue Medication
- Ease of Use: Sublingual sprays are straightforward to administer, as they involve spraying the medication under the tongue.
- Rapid Absorption: The sublingual mucosa has a rich blood supply, allowing for quick absorption of epinephrine into the bloodstream.
- Absorption Ready: Unlike sublingual films which require saliva for dissolution prior to absorption, epinephrine in the sublingual sprays is absorbed immediately upon administration, unaffected by saliva production.
- Reduced Anxiety: This method can be less intimidating for those with needle phobia, promoting better adherence and quicker administration in emergencies
The epinephrine sublingual formulation demonstrates exceptional stability, even at elevated temperatures up to 60℃/140°F, with no signs of degradation. It also resists freezing at temperatures as low as -20℃/-4°F. This lack of temperature sensitivity across a broad range of conditions, covering extreme summer and winter temperatures, ensures that the epinephrine sublingual spray can be used confidently by at-risk patients and their caregivers under any weather conditions. This is in stark contrast to auto-injectors and nasal sprays, which may experience potency drops under high temperatures or product failures in cold conditions due to possible drug solution freezing.
The unprecedented stability of the epinephrine sublingual spray also translates into an expected shelf life of two years or more, significantly reducing the need for frequent replacements that are common with auto-injectors or nasal sprays. This extended shelf life substantially lowers the financial burden for patients.
We believe that, if approved, our epinephrine sublingual spray will enable individuals suffering from severe allergies to live their best possible lives with the freedom and confidence to go wherever they want and do whatever they desire. By providing an alternative to the “much-dreaded” epinephrine auto-injectors, people living with anaphylaxis could finally experience a normal life, similar to everyone else. This development is not only empowering but also liberating for those managing life-threatening allergies.
Expanded Access Policy
Insignis Therapeutics is committed to developing promising new therapies to address the unmet medical needs of patients suffering from rare and seriously debilitating diseases.
We currently have late stage investigational medicines in our product pipeline for the treatment of severe allergic reactions (Type I) to insect stings or bites, foods, drugs, and other allergens as well as anaphylaxis of unknown cause (idiopathic anaphylaxis) or exercise induced anaphylaxis. Our goal is to provide access to our medicines at the appropriate time and in a manner that is most beneficial to the relevant patient population. We believe enrollment in our ongoing clinical trials is the safest and most effective way of achieving this goal.
We do recognize that some patients will not be eligible for our clinical trials and may wish to access our products through expanded access. However, at this time, we do not have the resources available to offer expanded access use of our investigational medicines.
We encourage all patients and physicians who are interested in accessing our investigational medicines to contact us using the Contact Us page of our website.
Insignis Therapeutics may revise this expanded access policy at any time. Additionally, the posting of this policy by Insignis Therapeutics does not serve as a guarantee of access to any specific investigational new drug by any individual patient.
If you have any questions, please reach out to us at mzhang@insignisrx.com. You can find further contact details on the Contact Us page of our website.
We may change this policy at any time. Please check back periodically for updates on EAP clinical trials.

